Authors
Grenville J. Croll & Ray Butler
Abstract
There is overwhelming evidence that the continued and widespread use of untested spreadsheets in business gives rise to regular, significant and unexpected financial losses.
Whilst this is worrying, it is perhaps a relatively minor concern compared with the risks arising from the use of poorly constructed and/or untested spreadsheets in medicine, a practice that is already occurring.
This article is intended as a warning that the use of poorly constructed and/or untested spreadsheets in clinical medicine cannot be tolerated.
It supports this warning by reporting on potentially serious weaknesses found while testing a limited number of publicly available clinical spreadsheets.
Sample
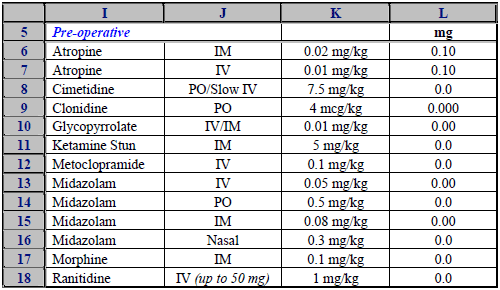
Our knowledge of the clinical domain was not sufficient to determine whether any material clinical errors were present.
However, we noted a very alarming incidence of poor / high risk practice in the spreadsheet modelling performed:
- Constants for drug dosage, risk factor scores, and predicted body measurements embedded in formulas.
- Complex nested IF formulas some with multiple AND and OR conditions. Many of these also had embedded constants.
- Protection / locked / unlocked cells.
- Formulas with no dependents – some were completeness checks, but some appeared to be potentially important calculations.
- Lack of documentation of the spreadsheets' workings, and instructions for their use was not present in the relevant Excel files.
- Lack of the use of data validation to ensure that accurate and appropriate data was input to the models.
- Most dosage information was given in milligrams. A very few doses were shown in micrograms (in the labels) but in a column headed mg (expressed in 3 decimals).
Publication
2006, EuSpRIG
